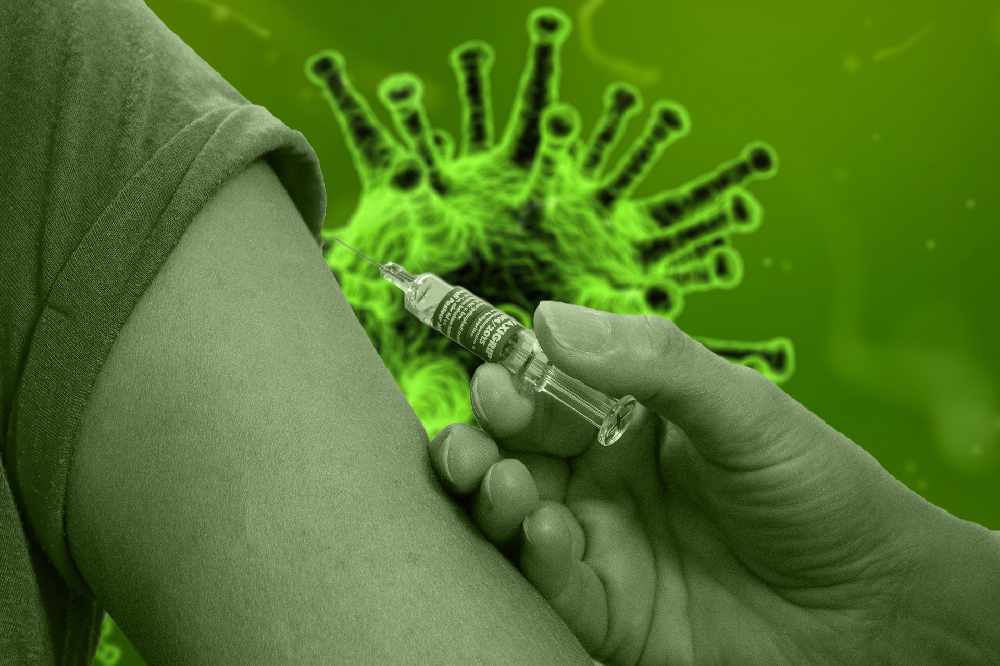
Spikevax shot approved for kids aged 6 to 11.
Moderna says Health Canada has approved its COVID-19 vaccine for use in children between the ages of 6 and 11.
The Spikevax shot was recently cleared for kids in the same age group in Australia and the EU.
The company is awaiting a decision from the FDA on the use of vaccine for children aged 12 to 17 in the United States.
Health Canada has approved two half doses for kids aged 6 to 11, taken one month apart.
Last year, Moderna said the vaccine generated virus-neutralizing antibodies for children in that age group.


 Taking Advantage of Staggering Tourism
Taking Advantage of Staggering Tourism
 Region to Address Transit Safety/Expansion
Region to Address Transit Safety/Expansion
 Be Wary of Increased Fraud/Scams: NRP
Be Wary of Increased Fraud/Scams: NRP
 NRP Help Bust Massive Auto Theft Ring
NRP Help Bust Massive Auto Theft Ring
 1st Greenhouse AGM Takes Place
1st Greenhouse AGM Takes Place
 City Taking Inquiries for Marineland: Mayor
City Taking Inquiries for Marineland: Mayor
 Niagara Wines Chosen by Politicians
Niagara Wines Chosen by Politicians
 Trees Planted in Dain City
Trees Planted in Dain City