
Drug maker to ask Health Canada to authorize COVID-19 shot for kids as young as five next week.
Pfizer is seeking approval in both Canada and the U.S. to allow use of the vaccine in kids aged five to 11.
Several media outlets report the drugmaker filed a formal request to the U.S. Food and Drug Administration today, and plans to submit a request to Health Canada in mid-October.
The FDA will meet on October 26th to discuss the issue.
Last week, Pfizer submitted data to both agencies to form a clinical trial on children in that age bracket.
The dose for younger children in the trial is about one-third the size of an adult shot.


 $3.5M of Crystal Meth seized from drug lab
$3.5M of Crystal Meth seized from drug lab
 NRP Searching for B&E Suspects
NRP Searching for B&E Suspects
 Welland CAO Optimistic for 2026
Welland CAO Optimistic for 2026
 Niagara Clinics Help Coughs, Colds
Niagara Clinics Help Coughs, Colds
 Clifton Hill Construction in January
Clifton Hill Construction in January
 Gas Prices to Hold Steady: Analyst
Gas Prices to Hold Steady: Analyst
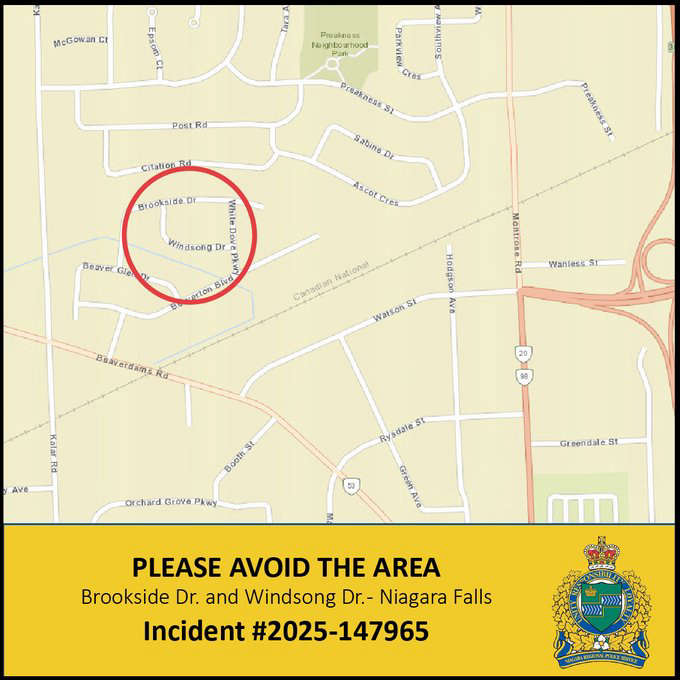 NRP Using Warrant in Niagara Falls Area
NRP Using Warrant in Niagara Falls Area
 Part of Welland Canal Officially Named
Part of Welland Canal Officially Named