Pfizer looking to become first company with approved COVID-19 vaccine for kids aged 5 to 11.
Pfizer has asked Health Canada to approve use of its vaccine on kids between the ages of 5 and 11.
If given the go-ahead, it'll be the first shot available to children in Canada, but new child-sized doses will need to be made available.
The vaccine was a team effort with Germany's BioNTech, and the doses are about one-third the size of that given to people 12 and older.
The company completed a clinical trial for its child-size dose, and the data was submitted to Health Canada earlier this month.
As well, a formal request for approval was made last week to the U.S. Food and Drug Administration.


 $3.5M of Crystal Meth seized from drug lab
$3.5M of Crystal Meth seized from drug lab
 NRP Searching for B&E Suspects
NRP Searching for B&E Suspects
 Welland CAO Optimistic for 2026
Welland CAO Optimistic for 2026
 Niagara Clinics Help Coughs, Colds
Niagara Clinics Help Coughs, Colds
 Clifton Hill Construction in January
Clifton Hill Construction in January
 Gas Prices to Hold Steady: Analyst
Gas Prices to Hold Steady: Analyst
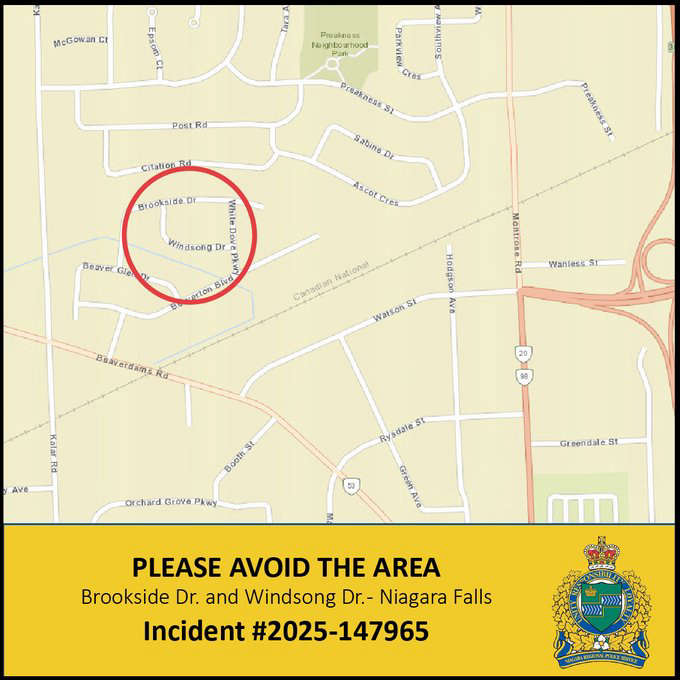 NRP Using Warrant in Niagara Falls Area
NRP Using Warrant in Niagara Falls Area
 Part of Welland Canal Officially Named
Part of Welland Canal Officially Named