Health Canada reviewing application from Moderna.
Federal officials say a decision about whether to approve Canada's first COVID-19 vaccine for babies and infants should be reached soon.
Deputy Chief Public Health Officer Howard NEW told reporters this morning, that Health Canada is still considering an application from Moderna.
The company has submitted a regulatory review to Health Canada for a vaccine to protect kids between six months and five years old.
The vaccine is two shots that are about a quarter of an adult dose, given about four weeks apart.
Earlier today, the FDA approved Moderna and Pfizer's vaccines for the youngest kids.


 Police Looking for Fraud Suspect
Police Looking for Fraud Suspect
 Suspect Charged with Murder
Suspect Charged with Murder
 Pedestrian Hit on Drummond Road
Pedestrian Hit on Drummond Road
 Late Chair Restored Council's Image: Rigby
Late Chair Restored Council's Image: Rigby
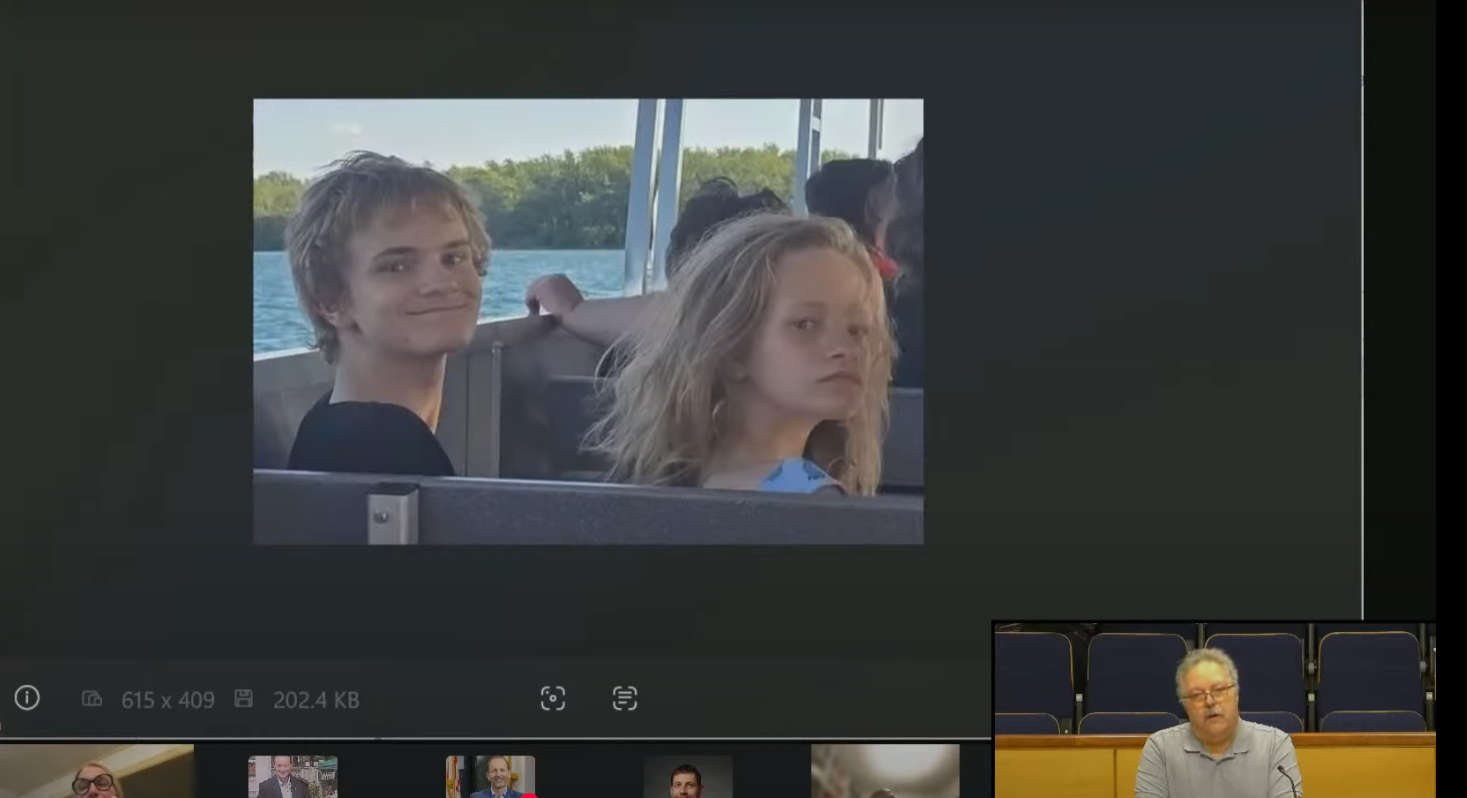 Water Safety Should be Taught in School: Region
Water Safety Should be Taught in School: Region
 OHIP Should Cover Prostate Screening: MPP
OHIP Should Cover Prostate Screening: MPP
 MPP Wants Greater Remembrance Day Observance
MPP Wants Greater Remembrance Day Observance
 Picture Released of Shooting Suspects
Picture Released of Shooting Suspects